Post by grrraaahhh on Sept 4, 2011 17:00:03 GMT -9
Preliminary Profile

NOMENCLATURE
Common Names. Polar bear, nanook, nanuq, nanuk, ice bear, sea bear, eisbar, isbj0rn, white bear.
Scientific Name. Ursus maritimus Phipps (1774) first described the polar bear as a species distinct from other bears and gave the name Ursus maritimus.
Subsequently, alternative generic names including Thalassarctos, Thalarctos, and Thalatarctos were suggested. Erdbrink (1953) and Thenius (1953) settled on Ursus (Thalarctos) maritimus, citing interbreeding between brown bears (Ursus arctos) and polar bears in zoos. Kurten (1964) described the evolution of polar bears based on the fossil record and recommended the name Ursus maritimus as adopted by Phipps (1774). Harington (1966), Manning (1971), and Wilson (1976) subsequently promoted use of the name Ursus maritimus, and it has predominated ever since.

Distribution and Population. Polar bears occur only in the Northern Hemisphere. Their range is limited to areas in which the sea is ice covered for much of the year. Over most of their range, polar bears remain on the sea-ice year-round or visit land only for short periods. Polar bears are common in the Chukchi and Beaufort Seas north of Alaska. They occur throughout the East Siberian, Laptev, and Kara Seas of Russia and the Barent's Sea of northern Europe. They are found in the northern part of the Greenland Sea, and are common in Baffin Bay, which separates Canada and Greenland, as well as through most of the Canadian Arctic Archipelago. Because their principal habitat is the sea-ice surface rather than adjacent land masses, they are classified as marine mammals. In most areas, pregnant females come ashore to create a den in which to give birth to young. Even then, however, they are quick to return to the sea ice as soon as cubs are able. In some areas, notably the Beaufort and Chukchi Seas of the polar basin, many females den and give birth to their young on drifting pack ice (Amstrup and Gardner 1994).
Polar bears are most abundant in shallow-water areas near shore and in other areas where currents and upwellings increase productivity and keep the ice cover from becoming too solidified in winter (Stirling and Smith 1975; Stirling et al. 1981; Amstrup and DeMaster 1988; Stirling 1990; Stirling and 0ritsland 1995; Stirling and Lunn 1997; Amstrup et al. 2000). Despite apparent preferences for the more productive waters near shorelines and polynyas (areas of persistent open water), polar bears occur throughout the polar basin including latitudes >88°N (Stefansson 1921; Papanin 1939; Durner and Amstrup 1995).
Because they derive their sustenance from the sea, the distribution of polar bears in most areas changes with the seasonal extent of sea-ice cover. In winter, for example, sea-ice extends as much as 400 km south of the Bering Strait, which separates Asia from North America, and polar bears extend their range to the southernmost extreme of the ice (Ray 1971). Sea-ice disappears from most of the Bering and Chukchi Seas in summer, and polar bears occupying these areas may migrate as much as 1000 km to stay with the southern edge of the pack ice (Garner et al. 1990, 1994). Throughout the polar basin, polar bears spend their summers concentrated along the edge of the persistent pack ice. Significant northerly and southerly movements appear to be dependent on seasonal melting and refreezing of ice near shore (Amstrup et al. 2000). In other areas, for example, Hudson Bay, James Bay, and portions of the Canadian High Arctic, when the sea-ice melts, polar bears are forced onto land for up to several months while they wait for winter and new ice (Jonkel et al. 1976; Schweinsburg 1979; Prevett and Kolenosky 1982; Schweinsburg and Lee 1982; Ferguson et al. 1997; Lunn et al.1997).

Until the 1960s, the prevalent belief was that polar bears wandered throughout the Arctic. Some naturalists felt that individual polar bears were carried passively with the predominant currents of the polar basin (Pedersen 1945). Researchers have known for some time that is not the case (Stirling et al. 1980, 1984). However the advent of radio telemetry (Amstrup et al. 1986), including the use of satellites (Fancy et al. 1988;
Harris et al. 1990; Messier et al. 1992; Amstrup et al. 2000), detailed knowledge of polar bear movements was not available.
EVOLUTION. The polar bear appears to share a common ancestor with the present-day brown bear. It apparently branched off the brown bear lineage during the late Pleistocene. Kurten (1964) suggested that ancestors of the modern polar bear were “gigantic.” Although still the largest of the extant bears, the polar bear, like many other mammals, has decreased in size since the Pleistocene. Also, significant morphological changes have continued within the last 20,000–40,000 years, perhaps through the present (Kurten 1964). Stanley (1979) described the many recently derived traits of polar bears as an example of “quantum speciation.”
Evidence of polar bear evolution contained in the sparse samples of fossils has been strengthened recently by molecular genetics. Whereas traits of fossil teeth and bones from polar bears clearly indicate their brown bear origins, fossil remains include only a handful of specimens (Kurten 1964). Genetic data from extant bears can provide phylogenetic information unavailable in the fossil record. Shields and Kocher (1991) first analyzed mtDNA sequences and showed a close relationship between brown bears and polar bears. Cronin et al. (1991) then discovered that mtDNA of brown bears is paraphyletic with respect to polar bears. That is, the mtDNA of brown bears of the Alexander Archipelago in southeastern Alaska is more closely related to the mtDNA of polar bears than it is to the mtDNA of other brown bears. Cronin et al. (1991) reported that mtDNA sequence divergence between Alexander Archipelago brown bears and polar bears is only about 1%, whereas a divergence of about 2.6% separates polar bears from brown bears occurring elsewhere. Cronin et al. (1991) and Cronin (1993) emphasized that mtDNA sequence divergence trees are not species trees and that mtDNA is not, by itself, a good measure of overall genetic differentiation. Nonetheless, these relationships provide a compelling argument regarding the origin and evolution of polar bears.

Following the discovery of Cronin et al. (1991), others corroborated the finding of paraphyletic mtDNA in brown bears and polar bears. Talbot and Shields (1996a, 1996b) suggested that the Alexander Archipelago brown bears represent descendents of ancestral stock that gave rise to polar bears. This stock may have survived Pleistocene glaciers in an ice-free refugium in southeastern Alaska, isolated from brown bears in other Pleistocene refugia (Heaton et al. 1996). This island-dwelling ancestral stock apparently has remained isolated from the more recent mainland bears by broad ocean passages.
Talbot and Shields (1996b) found mtDNA sequence divergence rates similar to those reported by Cronin et al. (1991), and proposed that ancestors of the Alexander Archipelago brown bears diverged from the other mtDNA lineages of brown bears 550,000–700,000 years ago.The mtDNA sequence divergences also suggested that polar bears branched from the Alexander Archipelago ancestral stock of brown bears about 200,000–250,000 years ago, a date closely corresponding with that suggested in the fossil record (Thenius 1953; Kurten 1964).Shields and Kocher (1991) and Cronin et al. (1991) reported that the mtDNA nucleotide sequence divergence between brown and polar bears (grouped together) and black bears was 7–9%. Applying the substitution rate (6%/million years) for mtDNA genes reported by Talbot and Shields (1996a) to the sequence divergence reported by Cronin et al. (1991) suggests that brown bear ancestral stock diverged from that of black bears approximately 1.2–1.5 million years ago. This “molecular clock” estimate may be low. The fossil record suggests black bears diverged from the brown bear lineage 1.5–2.5 million years ago.
Cronin (1993) cautioned that mutation rates vary among genes as well as among taxa, and that conclusions based on “molecular clocks” must be viewed with caution and in the context of other evidence. For example, mtDNA sequences for two functional nuclear genes, K-casein and the DQß gene of the major his to compatibility complex, show polyphyletic relationships among the three species of bears (M. Cronin and S. Amstrup, unpublished data). That is, the DNA sequences do not resolve the relationships among the species. These functional genes are presumably under strong selection and do not diverge as rapidly as mtDNA. Nonetheless, the mtDNA analyses indicate that Alexander Archipelago brown bear derive from more ancient stocks and are more closely related to polar bears than are other members of the brown bear clan. These conclusions also corroborate the recent appearance of the polar bear in the fossil record and the more ancient roots of the black bear (Thenius 1953; Kurten 1964). All DNA evidence, regardless of some areas of uncertainty, corroborate conclusions from the fossil record that the polar bear is a recently derived species and is undergoing rapid evolution. The extreme arctic marine environment is undoubtedly exerting strong selection pressures for rapid adaptation.
FEEDING HABITS. The polar bear is more predatory than other bears and is the apical predator of the arctic marine ecosystem. Polar bears prey heavily throughout their range on ringed seals (Phoca hispida) and, to a lesser extent, bearded seals (Erignathus barbatus). Ringed seals apparently have been a principal food of polar bears for a significant portion of their co evolutionary history and ringed seal behaviors appear to be oriented around avoidance of polar bear predation. Stirling (1977) contrasted the behavioral ecology of ringed seals and Weddell seals (Leptonychotes weddelli). Steady predation pressure from polar bears may have led ringed seals to use sub nivian birthing lairs and to interrupt spring and summer basking with frequent periods of scanning their surroundings for predators. Weddell seals, on the other hand, evolved in the Antarctic system, where surface predators are absent. They give birth unsheltered on the surface of the sea ice, and they are so ambivalent about activities on the ice surface that human researchers often can walk right up to them for study purposes (Stirling 1977).

Although seals are their primary prey, polar bears also have been known to kill much larger animals such as walruses (Odobenus rosmarus) and belugas (Delphinapterus leucas) (Stirling and Archibald 1977; Kiliaan et al. 1978; Smith 1980, 1985; Lowry et al. 1987; Calvert andStirling1990).The heaviest prey may be taken mainly by large male polar bears (Stirling and Derocher 1990), and unusual circumstances may be required. Nonetheless, in some areas and under some conditions, alternate prey may be quite important to polar bear sustenance. Stirling and Øritsland (1995) suggested that in areas where the estimated numbers of ringed seals are proportionately reduced relative to numbers of polar bears, other prey species were being substituted.
Overall, polar bears are most effective predators of young ringed seals, perhaps because they are naive with regard to predator avoidance. In spring, polar bears may concentrate their predatory efforts on capture of new-born ringed seal pups (Smith and Stirling 1975; Smith 1980). In some areas, predation on pups is extensive. Hammill and Smith (1991) estimated that polar bears annually kill up to 44%of newborn seal pups if conditions are right. Throughout the rest of the year, polar bears take seals predominantly from the first two year classes (Stirling et al. 1977a; Smith 1980). Whereas abundance of ringed seals may regulate density of polar bears in some areas, polar bear predation may regulate density and reproductive success of ringed seals in other areas (Hammill and Smith 1991; Stirling and Øritsland 1995).


The diet of the polar bear is comprised of more than 95 % seal,
mainly ringed seals or other smaller sized seals, as in Davis Strait
where the harp seal is much more abundant than ringed - ©
Ian Stirling
Polar bears apparently digest fat more easily than protein (Best 1984). They seem to prefer the fatty portions of seals (and presumably other animals) to muscle and other tissues. Stirling (1974) reported that polar bears often remove the fat layer from beneath the skin of freshly killed seals and consume it immediately. Because over half of the calories in a whole seal carcass may be located in the layer of fat between the skin and underlying muscle (Stirling and McEwan 1975), a bear that quickly consumes most of the fat available has maximized its caloric return in the minimal amount of time possible. This may be important to all but the largest polar bears because there is considerable competition for kills. Younger and smaller bears often are driven away from their kills by larger bears.
A high-fat and low-protein diet apparently serves polar bears physiologically as well. They are very efficient at recycling nitrogenous products of canabolism, and can use metabolic water released from fat metabolism (Nelson et al. 1983). Digestion of protein requires water, whereas digestion of fat releases water. In a cold environment, free water is available only at the energetic cost of melting ice and snow. The lipophilic habits of the polar bear minimize energy expended to obtain water in winter (Nelson 1981).

Polar bears tend not to cache prey animals they have killed like grizzly bears do (Stirling 1974; DeMaster and Stirling 1981; Stirling and Derocher 1990). This may be another reason why they consume the highest reward portion of their prey first. Although they have not been observed to cache, polar bears are surplus killers. Stirling and Derocher (1990) reported seeing a polar bear kill two seals with in an hour of feeding extensively on another seal. Neither of the latter two seals killed was eaten. Stirling and Øritsland (1995) also have reported surplus killing in polar bears. I once observed a young male polar bear still-hunting at a breathing hole on new autumn ice. There was a partially consumed seal nearby, and between that feeding site and where he was still-hunting were three freshly killed ringed seals stacked like cordwood. When my helicopter approached the bear to capture him, he abandoned his still-hunting site, ran to the pile of dead seals, and covered them with his body as if to protect his stash. This bear apparently had eaten his fill from the first seal but was continuing to hunt, catch, and stack seals despite a low probability that he would consume much of them.
An interesting adaptation to the carnivorous diet, and a difference between polar bears and other temperate and arctic bears, is that only the pregnant females enter dens for the entire winter. Other members of the population continue to hunt seals on the sea-ice throughout the winter. The year-around availability of seals allows denning in polar bears to be strictly a reproductive strategy (affording an acceptable environment for neonates), whereas in most bears it is largely a foraging strategy (avoiding the winter period of food unavailability).

Like other ursids, polar bears will eat human refuse (Lunn and Stirling 1985), and when trapped on land for long periods they will consume coastal marine and terrestrial plants and other terrestrial foods (Derocher et al. 1993). The significance of other foods to polar bears maybe limited, however (LunnandStirling1985; Derocher et al.1993). Over most of their range, polar bears have little opportunity to take foods of shoreline or terrestrial origin. Derocher et al. (1993) found that 31% of pregnant polar bears in the Hudson Bay area fed on berries before denning in autumn. The significance of this to their productivity was not known. Ramsay and Hobson (1991) and Hobson and Stirling (1997) differed in opinions of the value of supplemental terrestrial food. In general, the significance of terrestrial foraging to polar bears is poorly understood.
Clearly the value of alternate foods for polar bears depends on their richness and digestibility. Polar bears are poorly equipped to consume and digest most plant parts (Bunnell and Hamilton 1983), and it seems likely that except for fruiting bodies, plants will contribute little to their energy balance. Lunn and Stirling (1985) found that polar bears using human refuse at a dump maintained their weight or lost less weight than bears not using anthropogenic foods. Some bears using the dump even gained weight, but the supplemental food did not appear to confer a reproductive advantage (Lunn and Stirling 1985). Derocher et al. (2000) reported that some polar bears in Svalbard have become adept at catching reindeer (Rangifer tarandus). Considering the high digestibility of meat, it seems plausible that if readily available, reindeer could be an important alternate food of polar bears. Likewise, in the Beaufort Sea, dozens of polar bears each year have developed a habit of gathering at the butchering sites of bowhead whales (Balaena mysticetus) that are killed by local Native people. The value of this alternate food is apparently great, as nearly every bear seen near whale carcasses in autumn is obese.
Size & Weight. Male polar bears weigh about 375-600 kilograms (825-1320 pounds) while occasional individuals may reach 800 kilograms (1760 lb). They sometimes exceed 250 centimeters (10 feet) in length, measured in a straight line from the tip of the nose to the tip of the tail, although most male polar bears are a bit shorter. They are roughly twice the size and weight of adult females, which weigh 200 to 350 kilograms (440-750 lb) and achieve an adult body length of about 190-220 centimeters (up to about 7 ft). Females first breed at four to six years of age and most often give birth to two cubs in snow dens on land (some cubs are born in dens on the sea ice). Cubs stay with their mothers for two and a half years before weaning which means that unless cubs die prematurely, females do not breed more frequently than every three years. Both sexes live twenty to twenty-five years and sometimes to over 30 years. Their primary prey is ringed seals and, to a lesser degree, bearded seals.
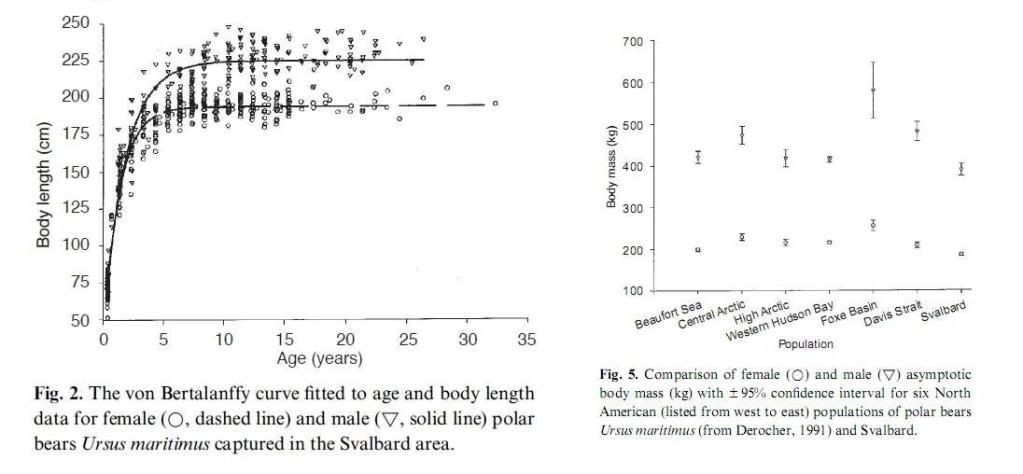
In Hudson Bay, the mean scale weight of 94 males >5 years of age was 489 kg. The largest bear in that group was a 13-year-old, which weighed 654 kg (Kolenosky et al. 1992). The heaviest bear we have weighed in Alaska was 610 kg, and several animals were heavy enough that we could not raise them with our helicopter or weighing tripod. Some animals too heavy to lift have been estimated to weigh 800 kg (DeMaster and Stirling 1981). Females are smaller, with peak weights usually not exceeding 400 kg. Total lengths of males in the Beaufort Sea of Alaska ranged up to 285 cm. Such an animal may reach nearly 4 m when standing on its hind legs and is 1.7 m shoulder height when standing on all four legs. Chest girth for large males is close to 200 cm. Although smaller, females in the Beaufort sea were as long as 247 cm with chest girths up to 175 cm. Only prehistoric polar bears and the giant short-faced bear (Arctodus spp.) of the Pleistocene were of greater stature than today's polar bears (Kurten 1964; Stirling and Derocher 1990).
Manning (1971) suggested there is a cline in size of polar bears across the Arctic. Size increases, he suggested, with distance from east Greenland across the Nearctic to the Chukchi Sea between Alaska and Russia. Manning (1971) also suggested that polar bears from Svalbard may be larger than those from east Greenland. A cline in size across the Palearctic also might occur, but samples from the Russian Arctic are inadequate to confirm it (Manning 1971).
The hypothesized cline was based on measurements made from skulls housed in museums around the world. Unfortunately, the sources of skulls in the various collections were not similar. Of particular note was that many of the skulls originating in the Chukchi Sea may have been donated by trophy hunters. These hunters worked over the ice in teams of aircraft (Tovey and Scott 1957) and were quite effective in killing a great number of the largest polar bears (Amstrup et al. 1986). Another potential problem is that ages of bears in the sample were estimated only by class or life stage. Hence, older bears from one locale might have been compared to younger bears (of the same age class) in another.
Potentially non standardized collection methods prevent any meaningful conclusions about relative sizes of polar bears from different locales. Also, if there is a cline in skull sizes around the world, it appears that body sizes and weights of polar bears do not follow a similar cline. The largest bears for which actual scale weights are known have come from the Hudson and James Bay areas of Canada and from the Beaufort Sea of Alaska, not from the Chukchi Sea. That observation, too, may be subject to some bias, as the most prolonged and intensive polar bear studies have been conducted in Hudson Bay and the Beaufort Sea. Greater numbers of captures in those locations may have increased the probability that very large bears were included in the sample.

Despite their large adult sizes, the young of polar bears are among the most altricial (undeveloped) of eutherian mammals (Ramsay and Dunbrack 1986). Newborn polar bears weigh only 600-700 g. They are blind, only lightly furred, and totally helpless (Blix and Lentfer 1979). Mother polar bears when giving birth commonly weigh over 300 kg, and can weigh 400 kg (Ramsay 1986). If only a single cub is born, the ratio of maternal to neonate weights could be between 400 and 500 to 1. Even with the more common two-cub litter, the ratio of maternal to neonate mass is extraordinarily large (Ramsay and Dunbrack 1986). Cubs grow very fast after birth. In Alaska, they average 13 kg on emergence from the den in late March or early April, with maximum weights of 22 kg. Cubs continue to grow rapidly through their first summer on the sea-ice and some weigh over 100 kg as they approach 1 year of age.
DENNING. Across most of their range, pregnant female polar bears excavate dens in snow and ice in early winter (Harington 1968; Lentfer and Hensel 1980; Ramsay and Stirling 1990; Amstrup and Gardner 1994). They give birth in those dens during midwinter (Kostyan 1954; Harington 1968; Ramsay and Dunbrack 1986) (see section on reproduction), and emerge from dens when cubs are approximately 3 months old. Because neonates are so altricial, the period of denning is essential to their early survival. Recognizing it as a critical phase in the polar bear life cycle, scientists have devoted much attention to aspects of maternal denning.
Denning on the Pack Ice. Although most maternal denning appears to occur on coastlines of main lands and islands, Amstrup and Gardner (1994) discovered that 53% of the dens of polar bears radio-collared between 1981 and 1991 were on drifting pack ice. They also found that 4% were on land-fast ice adjacent to shore. Lentfer and Hensel (1980) recognized the occurrence of dens on pack ice, but suggested that it was limited to bears that could not make it to shore to den. Harington (1968) concluded that denning on ice was not preferred, and Messier et al. (1994) reported no maternal denning on pack ice, although some “shelter denning” on pack ice was observed. The discovery that half of the bears in the Beaufort Sea may den on drifting sea ice, therefore, was not expected.

Claws. The claws of polar bears are shorter and more strongly curved than those of brown bears. They also are larger and heavier than those of black bears (Ursus americanus).They appear to be very well adapted to clambering over blocks of ice and snow and especially to securely gripping prey animals. The claws are normally black, but rarely may, like polar bear fur, lack pigment.
Skull and Dentition. Polar bears share the general ursid dental formula: I 3/3, C 1/1, P 4/4, M 2/3. The first premolars are vestigial and occur in a long diastema or gap between the functional canine and molari form teeth. That gap allows the powerful canines to penetrate deeply into the bodies of seals and other prey without interference from adjacent cheek teeth. Although polar bears apparently evolved from brown bears <250,000 years ago, their teeth have changed significantly from the brown bear form. The cheek teeth are greatly reduced in size and surface area, and the carnassials are more pronounced than in brown bears, reflecting the predatory lifestyle. The teeth of polar bears are well suited to the tasks of grabbing and holding prey and shearing meat and hide. They no longer are as suited to grinding grasses and other vegetation as are those of brown bears. The canine teeth of males are larger and heavier, relative to the size of the jaw, than those of females (Kurten 1955), and the molar arcade of males is longer than in females (Larsen).
Pelage. Polar bears are completely furred except for the tip of the nose. Pelage density is more even than in other ursids, which are often more sparsely furred ventrally and in axillary and groin areas. Even the pads of the feet of polar bears may be covered with hair, especially in late winter. Fur red foot pads may provide a more secure purchase on the slippery sea ice surface and add another layer of insulation between the bear’s foot and the substrate of ice and snow. Under the fur, pads of the feet of polar bears are made up of the same cornified epidermis characteristic of the pads of other bears (Storer and Tevis 1955; Ewer 1973).
The skin of polar bears is uniformly black. Hence, if polar bears lose hair due to physical trauma or disease, they appear from a distance to have black patches on their bodies. Polar bear fur appears white when it is clean and in even sunlight. Because it actually is without pigment, however (Øritsland and Ronald 1978; Grojean et al. 1980), bears may take on the yellow-orange hues of the setting and rising sun and the blue of sunlight filtered through clouds and fog. They appear the whitest right after molting. In spring and late winter, however, many polar bears are “off-white” or yellowish because of oils from their prey and other impurities that have attached to and been incorporated into their hair.
The molt appears to be some what variable, but begins by late April and May. The molt appears to be complete by late summer, and bears captured in autumn have notably shorter coats than those captured in spring. The pelt is thick with a dense under fur and guard hairs of various lengths. Polar bear fur may have a high propensity to take on the colors of environmental impurities because the guard hairs have a hollow medulla (or core) where impurities may lodge. In zoo environments, some species of algae can enter the hollow cores of guard hairs and result in a pronounced “greening” of the fur (Lewin and Robinson 1979).

in a pronounced “greening” of the fur (Lewin and Robinson 1979). Lavigne and Oritsland (1974) noted that polar bears effectively absorb ultraviolet (UV) light, and suggested that could be useful in remote-sensing surveys to enumerate them. The discovery that polar bears appear to absorb UV light led to much speculation about their ability to capture the energy in that light. Popular and scientific reports claimed that the ability to absorb energy in the UV spectrum was an adaptation to help maintain body heat in the rigorous Arctic environment (Anonymous 1978; Grojean et al. 1980; Lopez 1986; Mirsky 1988).Suddenly, the hollow hairs of polar bears, adept at catching algae and other contaminants (LewinandRobinson1979) also were endowed with the powers of optic fibers to funnel UV light to the skin .According to this theory, the skin was black to better absorb such energy without damage. Capturing this high-frequency electromagnetic energy would be a great adaptation for polar bears. This ability has attained the status of an Arctic legend, and contributed to the mystique surrounding the great white bears of the north.
Unfortunately, this supposed adaptation has no basis in fact. Lavigne (1988) and Koon (1998) established unequivocally that the hair of polar bears, although transparent in the visible spectrum, absorbs UV light. If the hair of polar bears absorbs UV light, it does not efficiently transmit UV light. As UV light moves down the shaft of the hair, its energy is absorbed, preventing significant energy from being transmitted to the skin.
REPRODUCTION. Reproduction in the female polar bear is similar to that in other ursids. They enter a prolonged estrus between March and June. In the polar basin, the peak of estrus as evidenced by turgidity of the vulva and vaginal discharge seems to be in late April and early May. Ovulation is thought to be induced by coitus (Wimsatt 1963; Ramsay and Dunbrack 1986; Derocher and Stirling 1992). Implantation is delayed until autumn, and total gestation is 195–265 days (Uspenski 1977), although during most of this time, active development of the conceptus is arrested. Young are born by early January (see below), but stay within the shelter of the den until March or early April (Amstrup and Gardner 1994). Litters of two cubs are most common over most of the polar bear range. Litters of three cubs are seen sporadically across the Arctic, and were most commonly reported in the Hudson Bay region (Stirling et al. 1977b; Ramsay and Stirling 1988; Derocher and Stirling 1992). Young bears will stay with their mothers until weaning most commonly in early spring when the cubs are 2.3 years of age. Female polar bears undergo a lactational anestrus and are available to breed again after weaning. Therefore, in most are as, the minimum successful reproductive interval for polar bears is 3 years (see below).
Newborn polar bears have hair, but are blind and weigh only 0.6 kg (Blix and Lentfer 1979). The growth of cubs is very rapid, and they may weigh 10–12 kg by the time they emerge from the den in the spring. After leaving the den, the rapid growth continues, and cubs may increase their weight by an order of magnitude between den exit and their & first birthday (S. C. Amstrup, unpublished data). Cubs can double their weight between their first and second birthdays. Cubs receive an especially rich milk from their mothers. The milk of polar bears typically has a higher fat content than that of other bears, and in general the milk of bears is richer in fat and protein than the milk of other carnivores (Jenness et al. 1972). Polar bear milk is more similar to that of pinnipeds than it is to milk of most terrestrial mammals (Jenness et al. 1972; Ramsay and Dunbrack 1986). Although polar bears may nurse cubs through their second birthday, some females apparently stop allowing cubs to suckle sometime after their first birthday. The contribution to growth from milk during the second year of life is much lower than during the first year (Arnould and Ramsay 1994). Arnould and Ramsay (1994) noted that fat content begins to decline fairly early in lactation, but the biggest differences are between the first and the second year of the cubs’s lives. Mean fat content of milk provided to cubs of the year was 31.2 ± 1.6%, whereas the fat content of milk fed to yearlings was 18.3 ± 2.4%. The energy contribution from milk is a significant contributor to the observed rapid growth of cubs and comes at a significant cost to mother bears (Arnould and Ramsay 1994).

The exact timing of birth may vary across the range of polar bears. Harington (1968) reported births as early as 30 November with a median date of 2 December. Derocher et al. (1992), reported, based on progesterone spikes in the blood of pregnant bears and the implied date of implantation, that births of Hudson Bay bears probably occur from mid-November through mid-December.
Messieretal.(1994) suggested that polar bears give birth by 15 December. In contrast, many pregnant female polar bears in the Beaufort Sea did not enter dens until late November or early December (Amstrup and Gardner 1994; S. C. Amstrup, unpublished data). Unless those bears were giving birth immediately on den entry, a later date of birth can be assumed. One captive female in Barrow, Alaska gave birth on 27 December, corroborating that assumption (Blix and Lentfer 1979). Similarly, Lønø (1972) reported that implantation of the conceptus into the uterus of the polar bear began in November, around the peak of den entry in the Beaufort Sea. The timing of implantation, and hence that of birth, is likely dependent on body condition of the female. Condition of the female, in turn, depends on a variety of environmental factors. The interaction between environmental and physiological factors that control births is clearly an area in need of further research.
Testes of male polar bears reside in the abdomen for most of the year. They descend into the scrotum in late winter, and remain there through May. Descent of the testes permits spermatogenesis, which is thought to occur from February to May (Erickson 1962; Lentfer and Miller 1969; Lønø 1970). Lønø (1970) reported that male/female pairs were observed as early as 8 March and as late as 20 June. According to histological examination of testes and ovaries, Lønø (1970) further concluded breeding could last into July. Deteriorating ice conditions preclude scientific observations in most polar bear habitats by June, so the frequency of summer breeding cannot be easily documented.
Lentfer and Miller (1969) concluded, from presence of mature spermatozoa in epididymides, that male polar bears in Alaska may be able to breed as early as 3 years of age. Presence of sperm also guaranteed reproductive capability until at least age 19 years (Lentfer and Miller 1969). A recent study in Greenland found that 2 of 7 two-yearold males, 5 of 10 three-year-olds, and 4 of 9 four-year-olds had some spermatazoa in epididymides (Rosing-Asvid et al. 2002). Although spermatazoa occurred at low density in the younger bears, all bears = 5 years old, except for one very thin individual, had produced abundant spermatozoa and appeared capable of breeding. Lentfer et al. (1980) observed males 3–11 years old in consort with estrous females, confirming at least the age of earliest breeding ability for male polar bears. It should be noted, however, that excessive hunting in Alaska just before and during the time those observations were made had all but eliminated prime males (aged >10years) from the population (Amstrupetal. 1986). Subsequently, few male bears that young have been observed with females. Since 1980, the proportion of prime males in Alaskan waters has been high (Amstrup 1995). Presently, large males weighing 400–500+ kg are abundant in this region. Three- and 4-year-old bears typically weigh = 250 kg, and would not be able to compete successfully for mates with the now-abundant large males. Currently, young males must have very low reproductive output despite their apparent reproductive potential.
Productivity of polar bear populations appears to be largely dependent on numbers and productivity of ringed seals. For example, in the Beaufort Sea, ringed seal densities are lower than in some areas of the Canadian High Arctic or Hudson Bay. As a possible consequence, female polar bears in the Beaufort Sea usually do not breed for the first time until they are 5 years of age (Stirling et al. 1976; Lentfer and Hensel 1980). This means they give birth for the first time at age 6. In contrast, across many areas of Canada, females reach maturity at age 4 and produce their first young at age 5 (Stirling et al. 1977b, 1980, 1984; Ramsay and Stirling 1982, 1988; Furnell and Schweinsburg 1984).
Craighead and Mitchell (1982:527) reported that in grizzly bears “reproductive longevity approximates physical longevity.” Female polar bears, on the other hand, may show a reproductive senescence long before the end of their lives. Derocheretal.(1992) calculated an average age of first breeding in the Hudson Bay area of 4.1 years. Productivity, assessed by estimated pregnancy rates, remained high between 5 and 20 years of age and declined thereafter (Derocher et al. 1992). Unfortunately, long-term monitoring of individual polar bears is uncommon and data addressing senescence are few. One 32-year-old female in the Beaufort Sea was monitored for the last 25 years of her life and seen annually during her last 10 years. This bear was in extraordinary condition nearly every autumn. Although she was not recaptured during the autumn of her 30th year, she was observed standing next to a 400-kg female that was captured that season. The 30-year-old female appeared larger, but still did not enter a den that autumn. Despite her apparent excellent physical condition, she last produced cubs at age 22, suggesting a prolonged reproductive senescence. Some contrary evidence also is available. One 29-year-old female in the Beaufort Sea was clearly in estrus (based on turgidity of the vulva) and traveling with an adult male in the spring of 2001. Derocher et al. (1992) also indicated that some females retained reproductive competency throughout life. The reproductive longevity of brown bears and polar bears appears to be fertile ground for further research.
Derocher and Stirling (1994) noted that litter size varied with maternal age, increasing until age 14 years, after which it declined. Heavy hunting reduces numbers of prime-age and older polar bears of both sexes (Amstrup et al. 1986). If such changes occurred without density-dependent increases in reproductive performance for young animals, over harvesting could have the additional population-depressing effect of actually reducing reproduction at low population densities rather than increasing it. Polar bears in the Hudson Bay area were heavily harvested into the 1970s, but numbers there appear to have increased since then (Prevett and Kolenosky 1982; Derocher et al. 1997). Litter size, litter production rate, and other reproductive factors can be expected to change with population size relative to carrying capacity. It also changes in a response to hunting pressure and other population perturbations. Hence, comparisons among populations or within populations over time must take into account the status of the population relative to natural and anthropogenic features of the environment.

In most parts of the Arctic, female polar bears cannot complete a reproductive cycle more frequently than every 3 years. The inter birth interval is determined by the length of time cubs are attended by their mothers, which most commonly is 2.3 years (Stirling et al. 1976, 1980; Lentfer et al. 1980; Amstrup et al. 1986; Amstrup and Durner 1995). Lønø (1970) concluded that in the Svalbard area, most cubs were weaned by about 17 months of age. Likewise, Ramsay and Stirling (1988) reported that during the 1970s and early 1980s, a significant proportion of female polar bears in the Hudson Bay region weaned their cubs at about 1.3 years of age. After weaning her cubs in the spring of their second year (at age 1.3 years), a female bear could breed again that same spring and achieve a 2-year reproductive interval.
The historically shorter reproductive interval of polar bears living in Hudson Bay (Stirlingetal.1977b) meant that they were more prolific than most other populations of polar bears. Captures of many hundreds of female polar bears and their young in Alaska, Canada, and Svalbard have suggested geographic differences in litter size, litter production, onset of maturity, and reproductive interval. For example, mean litter sizes of cubs and yearlings in Alaska were 1.63 and 1.49, respectively (Amstrup 1995). In Svalbard, these values were 1.81 and 1.32, respectively, whereas litter sizes of polar bears in Hudson Bay during the early 1980s were 1.9 and 1.7 for cubs and yearlings, respectively (Ramsay and Stirling 1988, Derocher and Stirling 1992). Annual litter production rates as high as 0.45 litters/female have been reported for polar bears in the Hudson Bay area (Derocher and Stirling 1992). Nearly half of the females in that population were annually producing a litter of cubs at that time. By comparison, only one fourth of the female polar bears in the Beaufort Sea produce a litter of cubs each year (a litter production rate of 0.25) (Amstrup 1995). That is, in Hudson Bay, each female had a litter nearly every other year, but in the Beaufort Sea, each female produced a litter only every fourth year. Because polar bears in Hudson Bay also produced larger litter sizes, these differences in litter production rates translated into a much higher overall reproductive rate there than in the Beaufort Sea. Female polar bears in the Beaufort Sea produced only ~ 0.40 cub/year, whereas in the Hudson Bay area they produced up to 0.90 cub/year at the time those studies were conducted (Derocher and Stirling 1992). Reproductive rates in most other areas appear to be more similar to those in the Beaufort Sea than in Hudson Bay.
In assessing reproductive intervals, it is critical to confirm weaning, as opposed to mortality of cubs. Many polar bear cubs die in their first year of life (Amstrup and Durner 1995). Those females can breed again in the year of the loss (if it occurs early enough in the spring) or the next year. The breeding frequency, by itself, might suggest a short reproductive interval when it is actually prolonged by poor cub survival. In addition to documenting that tagged females were no longer accompanied by yearling cubs in the spring, Ramsay and Stirling (1988) also captured many weaned yearlings in the autumn of their second year (approximately 1.8 years of age), confirming that many females in the Hudson Bay region actually did have a 2-year reproductive interval.

Lønø (1970), Stirling et al. (1977b), and Ramsay and Stirling (1988) reported on populations that may have been well below carrying capacity due to unregulated hunting (Stirling et al. 1977b; Larsen 1986; Derocher and Stirling 1992, 1995a). Likewise, breeding intervals in the Hudson Bay area have increased, possibly in response to increased relative density of bears in the area (Derocher and Stirling 1992, 1995b). Annual litter production rate in the Hudson Bay region declined from 0.45 litter/female in the period from 1965–1979 to 0.35 during 1985– 1990 (Derocher and Stirling 1992). A higher proportion produced cubs every 3 years in the latter period. The inverse of the litter production rate is the interbirth interval. That increased from 2.22 years in 1965– 1979 to 2.86 years in 1985–1990. Simultaneously, cub mortality from spring to autumn was significantly higher in the latter period (Derocher and Stirling 1992). The proximate factor associated with all of these trends was the declining weight of adult females during this 25-year period (Derocher and Stirling 1992). Age of first successful reproductive effort increased, although pregnancy rates did not change noticeably. An increasing age of maturation may indicate that a population is approaching carrying capacity. Age of maturation in mammals is often associated with attainment of a threshold body mass (Sadleir 1969) which could be more difficult to attain as competition for resources increases.
A delay in reaching that threshold mass may signal density dependent influences on the population. Such influences, however, also could result from environmental changes that reduce carrying capacity rather than from increases in polar bear numbers. The documented declines in body weights of females, declines in numbers of independent yearlings, and protracted reproductive intervals appear to be closely related to earlier deterioration of the sea-ice of Hudson Bay (Stirling etal.1999).Thesea-ice extent in the Arctic has been declining throughout the past two decades (Gloersen and Campbell 1991; Vinnikov et al. 1999). Declining Arctic sea-ice cover by itself is difficult to link with polar bear reproductive performance. The timing of melt of the sea-ice in Hudson Bay, however, is more easily connected. Polar bears there, especially pregnant females, depend heavily on the spring and early summer foraging for seals to carry them through the ice-free period (late summer to autumn). Pregnant females, unlike other polar bears in Hudson Bay, remain ashore in autumn when ice returns, and may be food deprived for up to 8 months. Those females must secure sufficient fat stores during the spring and summer to see them through that long period of food deprivation (Stirling 1977; Derocher and Stirling 1992). The mean date of sea-ice break-up in the late 1990s was >2 weeks earlier than it was in the 1970s and early 1980s (Stirling et al. 1999). Earlier break-up and the shortened foraging period accompanying it may mean a significant reduction in the fat stores female polar bears can accumulate before denning. This hypothesis is strengthened by the observation of a transient increase in condition of females coming ashore during the early 1990s when cooler than normal temperatures resulted in later break-ups (Stirling et al. 1999).
Evidence of the critical link between availability of seal prey and reproduction in polar bears is also available in more northerly parts of the range. Weights of females and their reproductive out put in the Beaufort Sea decreased markedly in the mid-1970s following a decline in ringed and bearded seal populations (Stirling et al. 1976, 1977b; Kingsley 1979; DeMaster et al. 1980; Stirling et al. 1982; Amstrup et al. 1986). The strength and longevity of declines in reproductive parameters varied both geographically and temporally with the severity of ice conditions that reduced numbers and productivity of seals (Amstrup et al. 1986).
Global warming. The Fourth Assessment Report of the Intergovernmental Panel on Climate Change of the United Nations, published in January 2007, came to the “unequivocal” conclusion that the world’s climate is warming rapidly and that the primary cause is human activity. What this means for polar bears, is that large-scale loss of ice will continue, with severe negative impacts on the bears’ survival, reproduction, and population size. The effects of climate warming will vary in timing and rate of change in different regions but, over the long term, the effects will be negative.

An adult female and her cub on shore in Svalbard. The ice is gone so now, like the bears in summer in Hudson Bay, they must live on her stored fat reserves for several months before they are able to return to the sea ice to hunt seals, a critical season witch duration may increase with global warming effects on arctic sea ice - © Ian Stirling.
In Hudson Bay-Foxe Basin and in the eastern Canadian Arctic (Baffin Bay and Davis Strait-Labrador Sea), the sea ice melts completely each summer. Since the most important feeding period for bears there is from mid-April until breakup, they are already among the first populations to be negatively affected by climate warming. Bears there survive the summer mainly on their stored fat, although they scavenge opportunistically, sometimes feed on vegetation, and may attempt to hunt other marine mammals. However, polar bears obtain almost all their annual energy requirements by hunting seals from the sea ice surface, not from other sources. Speculation that polar bears might sustain themselves from alternate food sources on land is fanciful.
The main area where multiyear ice presently remains in late summer and fall, and will in future, is along the coastline and between some of the most northerly islands of Canada and Greenland. If the relatively unproductive multiyear ice there is replaced by annual ice, over the continental shelf at least, it is possible that the biological productivity, and its suitability for seals and polar bears, may increase, at least in the short-term. However, if the climate continues to warm unchecked, as is currently predicted, this possible respite may be brief.

Despite the enormous changes in the Arctic that have already been documented, humans still can and must respond. It will likely be several decades before the most negative predictions are realized. Thus, time is critical for both polar bears and the sea ice that dominates the Arctic’s marine ecosystem. Polar bears evolved into existence because of a large productive habitat, unoccupied by a terrestrial predator. As that habitat disappears, so will the bears that live and depend on it. It is vital that all humans use whatever time remains to reduce greenhouse gas emissions sufficiently for sea ice and polar bears to persist for our children and grandchildren to marvel at. Nothing less is defensible.
Conservation History
In the 1960s and 1970s, hunting was the major threat to polar bears. Pressure from hunters was so great, and their survival in such jeopardy, that the five polar bear nations reached a landmark accord.
The International Agreement on the Conservation of Polar Bears was signed in Oslo, November 15, 1973 by: Canada, Denmark (Greenland), Norway, the U.S., and the former U.S.S.R. It is one of the first and most successful international conservation measures enacted in the 20th century.
The Agreement
* Prohibited random, unregulated sport hunting of polar bears
* Outlawed hunting from aircraft and icebreakers, a common practice
* Required each nation to protect polar bear denning areas and migration patterns
* Obliged the nations to conduct research on the conservation and management of polar bears to share research findings with each other
Today, member scientists from each nation continue to work together to address new threats including climate change, pollution, industrial activities, and poaching. They meet every three to four years under the auspices of the IUCN World Conservation Union to coordinate research.

Current Status
As of May 2008 the U.S, lists the polar bear as a threatened species under the Endangered Species Act. Russia considers the polar bear a species of concern.
What’s happening? Today, scientists have concluded that the threat to polar bears is ecological change in the Arctic from global warming. Polar bears depend on sea ice for hunting, breeding, and in some cases, denning. Summer ice loss in the Arctic now equals an area the size of Alaska, Texas, and the state of Washington combined.
Polar bears range from Russia to Alaska, from Canada to Greenland, and onto Norway's Svalbard archipelago—the five polar bear nations. Biologists estimate there are 20,000 to 25,000 bears. About 60% of those live in Canada.
At the 2009 meeting of the IUCN Polar Bear Specialist Group, scientists reported that of the 19 subpopulations* of polar bears:
* 8 are declining
* 3 are stable
* 1 is increasing
By comparison, in 2005:
* 5 were declining
* 5 were stable
* 2 were increasing
*Insufficient data to determine the fate of the other 7 populations

References
Amstrup, C. Steven. 1988. Polar Bears (Chapter 27) in Wild Mammals of North America.
PDF: www.polarbearsinternational.org/sites/default/files/pdf/PolarBearsComprehensive.pdf
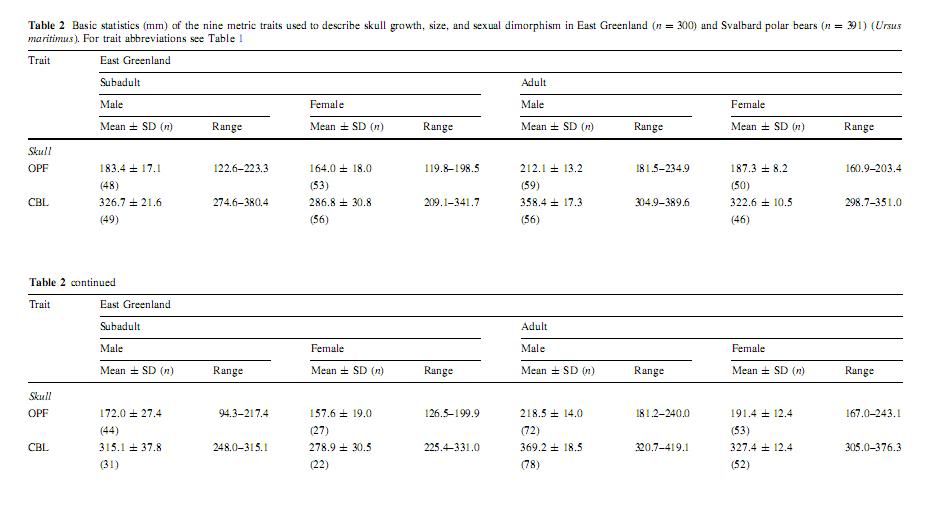
Bechshøft, Thea Ø, Sonne, Christian, Rigét, Frank F, Wiig, Øystein and Dietz, Rune. 2008. Differences in growth, size and sexual dimorphism in skulls of East Greenland and Svalbard polar bears (Ursus maritimus).
Derocher, A. E. and Ø. Wiig. 2002. Postnatal growth in body length and mass of polar bears (Ursus maritimus) at Svalbard.
Kolensky, B. George. 1987. POLAR BEAR (Chapter 37) in Wild Furbearer Management and Conservation in North America.
Manning, T. 1971. Geographical variation in the polar bear Ursus maritimus Phipps.
Polar Bears International: www.polarbearsinternational.org/polar-bears/bear-essentials-polar-style/conservation
Stirling, Ian. Polar Bears. 1988. University of Michigan Press Ann Arbor.
- Ours blanc: chronique d'une extinction annoncée: www.lecerclepolaire.com/articles_archives/Stirling-Polar_Bear.html
WWF: www.worldwildlife.org/species/finder/polarbear/polarbear.html
PDF LINKS ANEX:
www.usgs.gov/newsroom/special/pol....dyCondition.pdf
www.cnr.uidaho.edu/wlf314/lecture_notes/Rode_etal_2010.pdf
General Polar Bear discussion thread:
shaggygod.proboards.com/index.cgi?action=display&board=northamericanpolarbear&thread=644&page=1

NOMENCLATURE
Common Names. Polar bear, nanook, nanuq, nanuk, ice bear, sea bear, eisbar, isbj0rn, white bear.
Scientific Name. Ursus maritimus Phipps (1774) first described the polar bear as a species distinct from other bears and gave the name Ursus maritimus.
Subsequently, alternative generic names including Thalassarctos, Thalarctos, and Thalatarctos were suggested. Erdbrink (1953) and Thenius (1953) settled on Ursus (Thalarctos) maritimus, citing interbreeding between brown bears (Ursus arctos) and polar bears in zoos. Kurten (1964) described the evolution of polar bears based on the fossil record and recommended the name Ursus maritimus as adopted by Phipps (1774). Harington (1966), Manning (1971), and Wilson (1976) subsequently promoted use of the name Ursus maritimus, and it has predominated ever since.

Distribution and Population. Polar bears occur only in the Northern Hemisphere. Their range is limited to areas in which the sea is ice covered for much of the year. Over most of their range, polar bears remain on the sea-ice year-round or visit land only for short periods. Polar bears are common in the Chukchi and Beaufort Seas north of Alaska. They occur throughout the East Siberian, Laptev, and Kara Seas of Russia and the Barent's Sea of northern Europe. They are found in the northern part of the Greenland Sea, and are common in Baffin Bay, which separates Canada and Greenland, as well as through most of the Canadian Arctic Archipelago. Because their principal habitat is the sea-ice surface rather than adjacent land masses, they are classified as marine mammals. In most areas, pregnant females come ashore to create a den in which to give birth to young. Even then, however, they are quick to return to the sea ice as soon as cubs are able. In some areas, notably the Beaufort and Chukchi Seas of the polar basin, many females den and give birth to their young on drifting pack ice (Amstrup and Gardner 1994).
Polar bears are most abundant in shallow-water areas near shore and in other areas where currents and upwellings increase productivity and keep the ice cover from becoming too solidified in winter (Stirling and Smith 1975; Stirling et al. 1981; Amstrup and DeMaster 1988; Stirling 1990; Stirling and 0ritsland 1995; Stirling and Lunn 1997; Amstrup et al. 2000). Despite apparent preferences for the more productive waters near shorelines and polynyas (areas of persistent open water), polar bears occur throughout the polar basin including latitudes >88°N (Stefansson 1921; Papanin 1939; Durner and Amstrup 1995).
Because they derive their sustenance from the sea, the distribution of polar bears in most areas changes with the seasonal extent of sea-ice cover. In winter, for example, sea-ice extends as much as 400 km south of the Bering Strait, which separates Asia from North America, and polar bears extend their range to the southernmost extreme of the ice (Ray 1971). Sea-ice disappears from most of the Bering and Chukchi Seas in summer, and polar bears occupying these areas may migrate as much as 1000 km to stay with the southern edge of the pack ice (Garner et al. 1990, 1994). Throughout the polar basin, polar bears spend their summers concentrated along the edge of the persistent pack ice. Significant northerly and southerly movements appear to be dependent on seasonal melting and refreezing of ice near shore (Amstrup et al. 2000). In other areas, for example, Hudson Bay, James Bay, and portions of the Canadian High Arctic, when the sea-ice melts, polar bears are forced onto land for up to several months while they wait for winter and new ice (Jonkel et al. 1976; Schweinsburg 1979; Prevett and Kolenosky 1982; Schweinsburg and Lee 1982; Ferguson et al. 1997; Lunn et al.1997).

Until the 1960s, the prevalent belief was that polar bears wandered throughout the Arctic. Some naturalists felt that individual polar bears were carried passively with the predominant currents of the polar basin (Pedersen 1945). Researchers have known for some time that is not the case (Stirling et al. 1980, 1984). However the advent of radio telemetry (Amstrup et al. 1986), including the use of satellites (Fancy et al. 1988;
Harris et al. 1990; Messier et al. 1992; Amstrup et al. 2000), detailed knowledge of polar bear movements was not available.
EVOLUTION. The polar bear appears to share a common ancestor with the present-day brown bear. It apparently branched off the brown bear lineage during the late Pleistocene. Kurten (1964) suggested that ancestors of the modern polar bear were “gigantic.” Although still the largest of the extant bears, the polar bear, like many other mammals, has decreased in size since the Pleistocene. Also, significant morphological changes have continued within the last 20,000–40,000 years, perhaps through the present (Kurten 1964). Stanley (1979) described the many recently derived traits of polar bears as an example of “quantum speciation.”
Evidence of polar bear evolution contained in the sparse samples of fossils has been strengthened recently by molecular genetics. Whereas traits of fossil teeth and bones from polar bears clearly indicate their brown bear origins, fossil remains include only a handful of specimens (Kurten 1964). Genetic data from extant bears can provide phylogenetic information unavailable in the fossil record. Shields and Kocher (1991) first analyzed mtDNA sequences and showed a close relationship between brown bears and polar bears. Cronin et al. (1991) then discovered that mtDNA of brown bears is paraphyletic with respect to polar bears. That is, the mtDNA of brown bears of the Alexander Archipelago in southeastern Alaska is more closely related to the mtDNA of polar bears than it is to the mtDNA of other brown bears. Cronin et al. (1991) reported that mtDNA sequence divergence between Alexander Archipelago brown bears and polar bears is only about 1%, whereas a divergence of about 2.6% separates polar bears from brown bears occurring elsewhere. Cronin et al. (1991) and Cronin (1993) emphasized that mtDNA sequence divergence trees are not species trees and that mtDNA is not, by itself, a good measure of overall genetic differentiation. Nonetheless, these relationships provide a compelling argument regarding the origin and evolution of polar bears.

Following the discovery of Cronin et al. (1991), others corroborated the finding of paraphyletic mtDNA in brown bears and polar bears. Talbot and Shields (1996a, 1996b) suggested that the Alexander Archipelago brown bears represent descendents of ancestral stock that gave rise to polar bears. This stock may have survived Pleistocene glaciers in an ice-free refugium in southeastern Alaska, isolated from brown bears in other Pleistocene refugia (Heaton et al. 1996). This island-dwelling ancestral stock apparently has remained isolated from the more recent mainland bears by broad ocean passages.
Talbot and Shields (1996b) found mtDNA sequence divergence rates similar to those reported by Cronin et al. (1991), and proposed that ancestors of the Alexander Archipelago brown bears diverged from the other mtDNA lineages of brown bears 550,000–700,000 years ago.The mtDNA sequence divergences also suggested that polar bears branched from the Alexander Archipelago ancestral stock of brown bears about 200,000–250,000 years ago, a date closely corresponding with that suggested in the fossil record (Thenius 1953; Kurten 1964).Shields and Kocher (1991) and Cronin et al. (1991) reported that the mtDNA nucleotide sequence divergence between brown and polar bears (grouped together) and black bears was 7–9%. Applying the substitution rate (6%/million years) for mtDNA genes reported by Talbot and Shields (1996a) to the sequence divergence reported by Cronin et al. (1991) suggests that brown bear ancestral stock diverged from that of black bears approximately 1.2–1.5 million years ago. This “molecular clock” estimate may be low. The fossil record suggests black bears diverged from the brown bear lineage 1.5–2.5 million years ago.
Cronin (1993) cautioned that mutation rates vary among genes as well as among taxa, and that conclusions based on “molecular clocks” must be viewed with caution and in the context of other evidence. For example, mtDNA sequences for two functional nuclear genes, K-casein and the DQß gene of the major his to compatibility complex, show polyphyletic relationships among the three species of bears (M. Cronin and S. Amstrup, unpublished data). That is, the DNA sequences do not resolve the relationships among the species. These functional genes are presumably under strong selection and do not diverge as rapidly as mtDNA. Nonetheless, the mtDNA analyses indicate that Alexander Archipelago brown bear derive from more ancient stocks and are more closely related to polar bears than are other members of the brown bear clan. These conclusions also corroborate the recent appearance of the polar bear in the fossil record and the more ancient roots of the black bear (Thenius 1953; Kurten 1964). All DNA evidence, regardless of some areas of uncertainty, corroborate conclusions from the fossil record that the polar bear is a recently derived species and is undergoing rapid evolution. The extreme arctic marine environment is undoubtedly exerting strong selection pressures for rapid adaptation.
FEEDING HABITS. The polar bear is more predatory than other bears and is the apical predator of the arctic marine ecosystem. Polar bears prey heavily throughout their range on ringed seals (Phoca hispida) and, to a lesser extent, bearded seals (Erignathus barbatus). Ringed seals apparently have been a principal food of polar bears for a significant portion of their co evolutionary history and ringed seal behaviors appear to be oriented around avoidance of polar bear predation. Stirling (1977) contrasted the behavioral ecology of ringed seals and Weddell seals (Leptonychotes weddelli). Steady predation pressure from polar bears may have led ringed seals to use sub nivian birthing lairs and to interrupt spring and summer basking with frequent periods of scanning their surroundings for predators. Weddell seals, on the other hand, evolved in the Antarctic system, where surface predators are absent. They give birth unsheltered on the surface of the sea ice, and they are so ambivalent about activities on the ice surface that human researchers often can walk right up to them for study purposes (Stirling 1977).

Although seals are their primary prey, polar bears also have been known to kill much larger animals such as walruses (Odobenus rosmarus) and belugas (Delphinapterus leucas) (Stirling and Archibald 1977; Kiliaan et al. 1978; Smith 1980, 1985; Lowry et al. 1987; Calvert andStirling1990).The heaviest prey may be taken mainly by large male polar bears (Stirling and Derocher 1990), and unusual circumstances may be required. Nonetheless, in some areas and under some conditions, alternate prey may be quite important to polar bear sustenance. Stirling and Øritsland (1995) suggested that in areas where the estimated numbers of ringed seals are proportionately reduced relative to numbers of polar bears, other prey species were being substituted.
Overall, polar bears are most effective predators of young ringed seals, perhaps because they are naive with regard to predator avoidance. In spring, polar bears may concentrate their predatory efforts on capture of new-born ringed seal pups (Smith and Stirling 1975; Smith 1980). In some areas, predation on pups is extensive. Hammill and Smith (1991) estimated that polar bears annually kill up to 44%of newborn seal pups if conditions are right. Throughout the rest of the year, polar bears take seals predominantly from the first two year classes (Stirling et al. 1977a; Smith 1980). Whereas abundance of ringed seals may regulate density of polar bears in some areas, polar bear predation may regulate density and reproductive success of ringed seals in other areas (Hammill and Smith 1991; Stirling and Øritsland 1995).


The diet of the polar bear is comprised of more than 95 % seal,
mainly ringed seals or other smaller sized seals, as in Davis Strait
where the harp seal is much more abundant than ringed - ©
Ian Stirling
Polar bears apparently digest fat more easily than protein (Best 1984). They seem to prefer the fatty portions of seals (and presumably other animals) to muscle and other tissues. Stirling (1974) reported that polar bears often remove the fat layer from beneath the skin of freshly killed seals and consume it immediately. Because over half of the calories in a whole seal carcass may be located in the layer of fat between the skin and underlying muscle (Stirling and McEwan 1975), a bear that quickly consumes most of the fat available has maximized its caloric return in the minimal amount of time possible. This may be important to all but the largest polar bears because there is considerable competition for kills. Younger and smaller bears often are driven away from their kills by larger bears.
A high-fat and low-protein diet apparently serves polar bears physiologically as well. They are very efficient at recycling nitrogenous products of canabolism, and can use metabolic water released from fat metabolism (Nelson et al. 1983). Digestion of protein requires water, whereas digestion of fat releases water. In a cold environment, free water is available only at the energetic cost of melting ice and snow. The lipophilic habits of the polar bear minimize energy expended to obtain water in winter (Nelson 1981).

Polar bears tend not to cache prey animals they have killed like grizzly bears do (Stirling 1974; DeMaster and Stirling 1981; Stirling and Derocher 1990). This may be another reason why they consume the highest reward portion of their prey first. Although they have not been observed to cache, polar bears are surplus killers. Stirling and Derocher (1990) reported seeing a polar bear kill two seals with in an hour of feeding extensively on another seal. Neither of the latter two seals killed was eaten. Stirling and Øritsland (1995) also have reported surplus killing in polar bears. I once observed a young male polar bear still-hunting at a breathing hole on new autumn ice. There was a partially consumed seal nearby, and between that feeding site and where he was still-hunting were three freshly killed ringed seals stacked like cordwood. When my helicopter approached the bear to capture him, he abandoned his still-hunting site, ran to the pile of dead seals, and covered them with his body as if to protect his stash. This bear apparently had eaten his fill from the first seal but was continuing to hunt, catch, and stack seals despite a low probability that he would consume much of them.
An interesting adaptation to the carnivorous diet, and a difference between polar bears and other temperate and arctic bears, is that only the pregnant females enter dens for the entire winter. Other members of the population continue to hunt seals on the sea-ice throughout the winter. The year-around availability of seals allows denning in polar bears to be strictly a reproductive strategy (affording an acceptable environment for neonates), whereas in most bears it is largely a foraging strategy (avoiding the winter period of food unavailability).

Like other ursids, polar bears will eat human refuse (Lunn and Stirling 1985), and when trapped on land for long periods they will consume coastal marine and terrestrial plants and other terrestrial foods (Derocher et al. 1993). The significance of other foods to polar bears maybe limited, however (LunnandStirling1985; Derocher et al.1993). Over most of their range, polar bears have little opportunity to take foods of shoreline or terrestrial origin. Derocher et al. (1993) found that 31% of pregnant polar bears in the Hudson Bay area fed on berries before denning in autumn. The significance of this to their productivity was not known. Ramsay and Hobson (1991) and Hobson and Stirling (1997) differed in opinions of the value of supplemental terrestrial food. In general, the significance of terrestrial foraging to polar bears is poorly understood.
Clearly the value of alternate foods for polar bears depends on their richness and digestibility. Polar bears are poorly equipped to consume and digest most plant parts (Bunnell and Hamilton 1983), and it seems likely that except for fruiting bodies, plants will contribute little to their energy balance. Lunn and Stirling (1985) found that polar bears using human refuse at a dump maintained their weight or lost less weight than bears not using anthropogenic foods. Some bears using the dump even gained weight, but the supplemental food did not appear to confer a reproductive advantage (Lunn and Stirling 1985). Derocher et al. (2000) reported that some polar bears in Svalbard have become adept at catching reindeer (Rangifer tarandus). Considering the high digestibility of meat, it seems plausible that if readily available, reindeer could be an important alternate food of polar bears. Likewise, in the Beaufort Sea, dozens of polar bears each year have developed a habit of gathering at the butchering sites of bowhead whales (Balaena mysticetus) that are killed by local Native people. The value of this alternate food is apparently great, as nearly every bear seen near whale carcasses in autumn is obese.
Size & Weight. Male polar bears weigh about 375-600 kilograms (825-1320 pounds) while occasional individuals may reach 800 kilograms (1760 lb). They sometimes exceed 250 centimeters (10 feet) in length, measured in a straight line from the tip of the nose to the tip of the tail, although most male polar bears are a bit shorter. They are roughly twice the size and weight of adult females, which weigh 200 to 350 kilograms (440-750 lb) and achieve an adult body length of about 190-220 centimeters (up to about 7 ft). Females first breed at four to six years of age and most often give birth to two cubs in snow dens on land (some cubs are born in dens on the sea ice). Cubs stay with their mothers for two and a half years before weaning which means that unless cubs die prematurely, females do not breed more frequently than every three years. Both sexes live twenty to twenty-five years and sometimes to over 30 years. Their primary prey is ringed seals and, to a lesser degree, bearded seals.


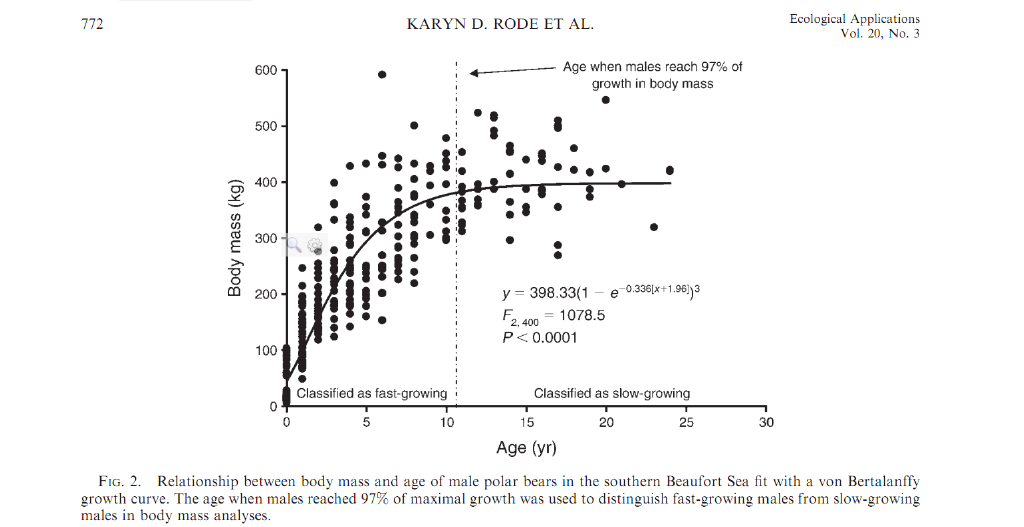
In Hudson Bay, the mean scale weight of 94 males >5 years of age was 489 kg. The largest bear in that group was a 13-year-old, which weighed 654 kg (Kolenosky et al. 1992). The heaviest bear we have weighed in Alaska was 610 kg, and several animals were heavy enough that we could not raise them with our helicopter or weighing tripod. Some animals too heavy to lift have been estimated to weigh 800 kg (DeMaster and Stirling 1981). Females are smaller, with peak weights usually not exceeding 400 kg. Total lengths of males in the Beaufort Sea of Alaska ranged up to 285 cm. Such an animal may reach nearly 4 m when standing on its hind legs and is 1.7 m shoulder height when standing on all four legs. Chest girth for large males is close to 200 cm. Although smaller, females in the Beaufort sea were as long as 247 cm with chest girths up to 175 cm. Only prehistoric polar bears and the giant short-faced bear (Arctodus spp.) of the Pleistocene were of greater stature than today's polar bears (Kurten 1964; Stirling and Derocher 1990).
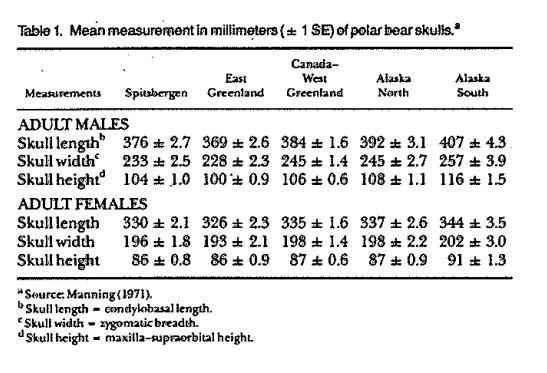
Manning (1971) suggested there is a cline in size of polar bears across the Arctic. Size increases, he suggested, with distance from east Greenland across the Nearctic to the Chukchi Sea between Alaska and Russia. Manning (1971) also suggested that polar bears from Svalbard may be larger than those from east Greenland. A cline in size across the Palearctic also might occur, but samples from the Russian Arctic are inadequate to confirm it (Manning 1971).
The hypothesized cline was based on measurements made from skulls housed in museums around the world. Unfortunately, the sources of skulls in the various collections were not similar. Of particular note was that many of the skulls originating in the Chukchi Sea may have been donated by trophy hunters. These hunters worked over the ice in teams of aircraft (Tovey and Scott 1957) and were quite effective in killing a great number of the largest polar bears (Amstrup et al. 1986). Another potential problem is that ages of bears in the sample were estimated only by class or life stage. Hence, older bears from one locale might have been compared to younger bears (of the same age class) in another.
Potentially non standardized collection methods prevent any meaningful conclusions about relative sizes of polar bears from different locales. Also, if there is a cline in skull sizes around the world, it appears that body sizes and weights of polar bears do not follow a similar cline. The largest bears for which actual scale weights are known have come from the Hudson and James Bay areas of Canada and from the Beaufort Sea of Alaska, not from the Chukchi Sea. That observation, too, may be subject to some bias, as the most prolonged and intensive polar bear studies have been conducted in Hudson Bay and the Beaufort Sea. Greater numbers of captures in those locations may have increased the probability that very large bears were included in the sample.

Despite their large adult sizes, the young of polar bears are among the most altricial (undeveloped) of eutherian mammals (Ramsay and Dunbrack 1986). Newborn polar bears weigh only 600-700 g. They are blind, only lightly furred, and totally helpless (Blix and Lentfer 1979). Mother polar bears when giving birth commonly weigh over 300 kg, and can weigh 400 kg (Ramsay 1986). If only a single cub is born, the ratio of maternal to neonate weights could be between 400 and 500 to 1. Even with the more common two-cub litter, the ratio of maternal to neonate mass is extraordinarily large (Ramsay and Dunbrack 1986). Cubs grow very fast after birth. In Alaska, they average 13 kg on emergence from the den in late March or early April, with maximum weights of 22 kg. Cubs continue to grow rapidly through their first summer on the sea-ice and some weigh over 100 kg as they approach 1 year of age.
DENNING. Across most of their range, pregnant female polar bears excavate dens in snow and ice in early winter (Harington 1968; Lentfer and Hensel 1980; Ramsay and Stirling 1990; Amstrup and Gardner 1994). They give birth in those dens during midwinter (Kostyan 1954; Harington 1968; Ramsay and Dunbrack 1986) (see section on reproduction), and emerge from dens when cubs are approximately 3 months old. Because neonates are so altricial, the period of denning is essential to their early survival. Recognizing it as a critical phase in the polar bear life cycle, scientists have devoted much attention to aspects of maternal denning.
Denning on the Pack Ice. Although most maternal denning appears to occur on coastlines of main lands and islands, Amstrup and Gardner (1994) discovered that 53% of the dens of polar bears radio-collared between 1981 and 1991 were on drifting pack ice. They also found that 4% were on land-fast ice adjacent to shore. Lentfer and Hensel (1980) recognized the occurrence of dens on pack ice, but suggested that it was limited to bears that could not make it to shore to den. Harington (1968) concluded that denning on ice was not preferred, and Messier et al. (1994) reported no maternal denning on pack ice, although some “shelter denning” on pack ice was observed. The discovery that half of the bears in the Beaufort Sea may den on drifting sea ice, therefore, was not expected.

Claws. The claws of polar bears are shorter and more strongly curved than those of brown bears. They also are larger and heavier than those of black bears (Ursus americanus).They appear to be very well adapted to clambering over blocks of ice and snow and especially to securely gripping prey animals. The claws are normally black, but rarely may, like polar bear fur, lack pigment.
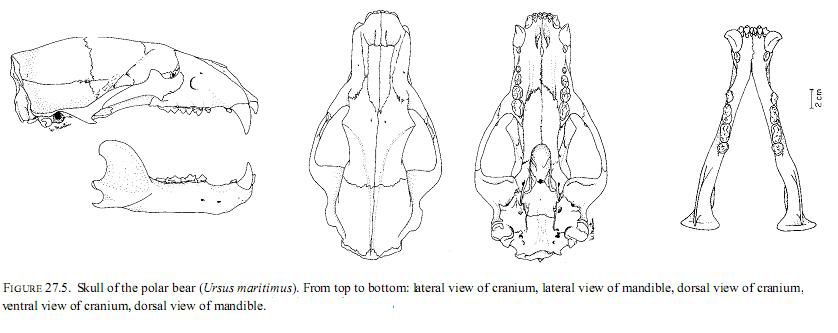
Skull and Dentition. Polar bears share the general ursid dental formula: I 3/3, C 1/1, P 4/4, M 2/3. The first premolars are vestigial and occur in a long diastema or gap between the functional canine and molari form teeth. That gap allows the powerful canines to penetrate deeply into the bodies of seals and other prey without interference from adjacent cheek teeth. Although polar bears apparently evolved from brown bears <250,000 years ago, their teeth have changed significantly from the brown bear form. The cheek teeth are greatly reduced in size and surface area, and the carnassials are more pronounced than in brown bears, reflecting the predatory lifestyle. The teeth of polar bears are well suited to the tasks of grabbing and holding prey and shearing meat and hide. They no longer are as suited to grinding grasses and other vegetation as are those of brown bears. The canine teeth of males are larger and heavier, relative to the size of the jaw, than those of females (Kurten 1955), and the molar arcade of males is longer than in females (Larsen).



Pelage. Polar bears are completely furred except for the tip of the nose. Pelage density is more even than in other ursids, which are often more sparsely furred ventrally and in axillary and groin areas. Even the pads of the feet of polar bears may be covered with hair, especially in late winter. Fur red foot pads may provide a more secure purchase on the slippery sea ice surface and add another layer of insulation between the bear’s foot and the substrate of ice and snow. Under the fur, pads of the feet of polar bears are made up of the same cornified epidermis characteristic of the pads of other bears (Storer and Tevis 1955; Ewer 1973).
The skin of polar bears is uniformly black. Hence, if polar bears lose hair due to physical trauma or disease, they appear from a distance to have black patches on their bodies. Polar bear fur appears white when it is clean and in even sunlight. Because it actually is without pigment, however (Øritsland and Ronald 1978; Grojean et al. 1980), bears may take on the yellow-orange hues of the setting and rising sun and the blue of sunlight filtered through clouds and fog. They appear the whitest right after molting. In spring and late winter, however, many polar bears are “off-white” or yellowish because of oils from their prey and other impurities that have attached to and been incorporated into their hair.
The molt appears to be some what variable, but begins by late April and May. The molt appears to be complete by late summer, and bears captured in autumn have notably shorter coats than those captured in spring. The pelt is thick with a dense under fur and guard hairs of various lengths. Polar bear fur may have a high propensity to take on the colors of environmental impurities because the guard hairs have a hollow medulla (or core) where impurities may lodge. In zoo environments, some species of algae can enter the hollow cores of guard hairs and result in a pronounced “greening” of the fur (Lewin and Robinson 1979).

in a pronounced “greening” of the fur (Lewin and Robinson 1979). Lavigne and Oritsland (1974) noted that polar bears effectively absorb ultraviolet (UV) light, and suggested that could be useful in remote-sensing surveys to enumerate them. The discovery that polar bears appear to absorb UV light led to much speculation about their ability to capture the energy in that light. Popular and scientific reports claimed that the ability to absorb energy in the UV spectrum was an adaptation to help maintain body heat in the rigorous Arctic environment (Anonymous 1978; Grojean et al. 1980; Lopez 1986; Mirsky 1988).Suddenly, the hollow hairs of polar bears, adept at catching algae and other contaminants (LewinandRobinson1979) also were endowed with the powers of optic fibers to funnel UV light to the skin .According to this theory, the skin was black to better absorb such energy without damage. Capturing this high-frequency electromagnetic energy would be a great adaptation for polar bears. This ability has attained the status of an Arctic legend, and contributed to the mystique surrounding the great white bears of the north.
Unfortunately, this supposed adaptation has no basis in fact. Lavigne (1988) and Koon (1998) established unequivocally that the hair of polar bears, although transparent in the visible spectrum, absorbs UV light. If the hair of polar bears absorbs UV light, it does not efficiently transmit UV light. As UV light moves down the shaft of the hair, its energy is absorbed, preventing significant energy from being transmitted to the skin.
REPRODUCTION. Reproduction in the female polar bear is similar to that in other ursids. They enter a prolonged estrus between March and June. In the polar basin, the peak of estrus as evidenced by turgidity of the vulva and vaginal discharge seems to be in late April and early May. Ovulation is thought to be induced by coitus (Wimsatt 1963; Ramsay and Dunbrack 1986; Derocher and Stirling 1992). Implantation is delayed until autumn, and total gestation is 195–265 days (Uspenski 1977), although during most of this time, active development of the conceptus is arrested. Young are born by early January (see below), but stay within the shelter of the den until March or early April (Amstrup and Gardner 1994). Litters of two cubs are most common over most of the polar bear range. Litters of three cubs are seen sporadically across the Arctic, and were most commonly reported in the Hudson Bay region (Stirling et al. 1977b; Ramsay and Stirling 1988; Derocher and Stirling 1992). Young bears will stay with their mothers until weaning most commonly in early spring when the cubs are 2.3 years of age. Female polar bears undergo a lactational anestrus and are available to breed again after weaning. Therefore, in most are as, the minimum successful reproductive interval for polar bears is 3 years (see below).
Newborn polar bears have hair, but are blind and weigh only 0.6 kg (Blix and Lentfer 1979). The growth of cubs is very rapid, and they may weigh 10–12 kg by the time they emerge from the den in the spring. After leaving the den, the rapid growth continues, and cubs may increase their weight by an order of magnitude between den exit and their & first birthday (S. C. Amstrup, unpublished data). Cubs can double their weight between their first and second birthdays. Cubs receive an especially rich milk from their mothers. The milk of polar bears typically has a higher fat content than that of other bears, and in general the milk of bears is richer in fat and protein than the milk of other carnivores (Jenness et al. 1972). Polar bear milk is more similar to that of pinnipeds than it is to milk of most terrestrial mammals (Jenness et al. 1972; Ramsay and Dunbrack 1986). Although polar bears may nurse cubs through their second birthday, some females apparently stop allowing cubs to suckle sometime after their first birthday. The contribution to growth from milk during the second year of life is much lower than during the first year (Arnould and Ramsay 1994). Arnould and Ramsay (1994) noted that fat content begins to decline fairly early in lactation, but the biggest differences are between the first and the second year of the cubs’s lives. Mean fat content of milk provided to cubs of the year was 31.2 ± 1.6%, whereas the fat content of milk fed to yearlings was 18.3 ± 2.4%. The energy contribution from milk is a significant contributor to the observed rapid growth of cubs and comes at a significant cost to mother bears (Arnould and Ramsay 1994).

The exact timing of birth may vary across the range of polar bears. Harington (1968) reported births as early as 30 November with a median date of 2 December. Derocher et al. (1992), reported, based on progesterone spikes in the blood of pregnant bears and the implied date of implantation, that births of Hudson Bay bears probably occur from mid-November through mid-December.
Messieretal.(1994) suggested that polar bears give birth by 15 December. In contrast, many pregnant female polar bears in the Beaufort Sea did not enter dens until late November or early December (Amstrup and Gardner 1994; S. C. Amstrup, unpublished data). Unless those bears were giving birth immediately on den entry, a later date of birth can be assumed. One captive female in Barrow, Alaska gave birth on 27 December, corroborating that assumption (Blix and Lentfer 1979). Similarly, Lønø (1972) reported that implantation of the conceptus into the uterus of the polar bear began in November, around the peak of den entry in the Beaufort Sea. The timing of implantation, and hence that of birth, is likely dependent on body condition of the female. Condition of the female, in turn, depends on a variety of environmental factors. The interaction between environmental and physiological factors that control births is clearly an area in need of further research.
Testes of male polar bears reside in the abdomen for most of the year. They descend into the scrotum in late winter, and remain there through May. Descent of the testes permits spermatogenesis, which is thought to occur from February to May (Erickson 1962; Lentfer and Miller 1969; Lønø 1970). Lønø (1970) reported that male/female pairs were observed as early as 8 March and as late as 20 June. According to histological examination of testes and ovaries, Lønø (1970) further concluded breeding could last into July. Deteriorating ice conditions preclude scientific observations in most polar bear habitats by June, so the frequency of summer breeding cannot be easily documented.
Lentfer and Miller (1969) concluded, from presence of mature spermatozoa in epididymides, that male polar bears in Alaska may be able to breed as early as 3 years of age. Presence of sperm also guaranteed reproductive capability until at least age 19 years (Lentfer and Miller 1969). A recent study in Greenland found that 2 of 7 two-yearold males, 5 of 10 three-year-olds, and 4 of 9 four-year-olds had some spermatazoa in epididymides (Rosing-Asvid et al. 2002). Although spermatazoa occurred at low density in the younger bears, all bears = 5 years old, except for one very thin individual, had produced abundant spermatozoa and appeared capable of breeding. Lentfer et al. (1980) observed males 3–11 years old in consort with estrous females, confirming at least the age of earliest breeding ability for male polar bears. It should be noted, however, that excessive hunting in Alaska just before and during the time those observations were made had all but eliminated prime males (aged >10years) from the population (Amstrupetal. 1986). Subsequently, few male bears that young have been observed with females. Since 1980, the proportion of prime males in Alaskan waters has been high (Amstrup 1995). Presently, large males weighing 400–500+ kg are abundant in this region. Three- and 4-year-old bears typically weigh = 250 kg, and would not be able to compete successfully for mates with the now-abundant large males. Currently, young males must have very low reproductive output despite their apparent reproductive potential.
Productivity of polar bear populations appears to be largely dependent on numbers and productivity of ringed seals. For example, in the Beaufort Sea, ringed seal densities are lower than in some areas of the Canadian High Arctic or Hudson Bay. As a possible consequence, female polar bears in the Beaufort Sea usually do not breed for the first time until they are 5 years of age (Stirling et al. 1976; Lentfer and Hensel 1980). This means they give birth for the first time at age 6. In contrast, across many areas of Canada, females reach maturity at age 4 and produce their first young at age 5 (Stirling et al. 1977b, 1980, 1984; Ramsay and Stirling 1982, 1988; Furnell and Schweinsburg 1984).
Craighead and Mitchell (1982:527) reported that in grizzly bears “reproductive longevity approximates physical longevity.” Female polar bears, on the other hand, may show a reproductive senescence long before the end of their lives. Derocheretal.(1992) calculated an average age of first breeding in the Hudson Bay area of 4.1 years. Productivity, assessed by estimated pregnancy rates, remained high between 5 and 20 years of age and declined thereafter (Derocher et al. 1992). Unfortunately, long-term monitoring of individual polar bears is uncommon and data addressing senescence are few. One 32-year-old female in the Beaufort Sea was monitored for the last 25 years of her life and seen annually during her last 10 years. This bear was in extraordinary condition nearly every autumn. Although she was not recaptured during the autumn of her 30th year, she was observed standing next to a 400-kg female that was captured that season. The 30-year-old female appeared larger, but still did not enter a den that autumn. Despite her apparent excellent physical condition, she last produced cubs at age 22, suggesting a prolonged reproductive senescence. Some contrary evidence also is available. One 29-year-old female in the Beaufort Sea was clearly in estrus (based on turgidity of the vulva) and traveling with an adult male in the spring of 2001. Derocher et al. (1992) also indicated that some females retained reproductive competency throughout life. The reproductive longevity of brown bears and polar bears appears to be fertile ground for further research.
Derocher and Stirling (1994) noted that litter size varied with maternal age, increasing until age 14 years, after which it declined. Heavy hunting reduces numbers of prime-age and older polar bears of both sexes (Amstrup et al. 1986). If such changes occurred without density-dependent increases in reproductive performance for young animals, over harvesting could have the additional population-depressing effect of actually reducing reproduction at low population densities rather than increasing it. Polar bears in the Hudson Bay area were heavily harvested into the 1970s, but numbers there appear to have increased since then (Prevett and Kolenosky 1982; Derocher et al. 1997). Litter size, litter production rate, and other reproductive factors can be expected to change with population size relative to carrying capacity. It also changes in a response to hunting pressure and other population perturbations. Hence, comparisons among populations or within populations over time must take into account the status of the population relative to natural and anthropogenic features of the environment.

In most parts of the Arctic, female polar bears cannot complete a reproductive cycle more frequently than every 3 years. The inter birth interval is determined by the length of time cubs are attended by their mothers, which most commonly is 2.3 years (Stirling et al. 1976, 1980; Lentfer et al. 1980; Amstrup et al. 1986; Amstrup and Durner 1995). Lønø (1970) concluded that in the Svalbard area, most cubs were weaned by about 17 months of age. Likewise, Ramsay and Stirling (1988) reported that during the 1970s and early 1980s, a significant proportion of female polar bears in the Hudson Bay region weaned their cubs at about 1.3 years of age. After weaning her cubs in the spring of their second year (at age 1.3 years), a female bear could breed again that same spring and achieve a 2-year reproductive interval.
The historically shorter reproductive interval of polar bears living in Hudson Bay (Stirlingetal.1977b) meant that they were more prolific than most other populations of polar bears. Captures of many hundreds of female polar bears and their young in Alaska, Canada, and Svalbard have suggested geographic differences in litter size, litter production, onset of maturity, and reproductive interval. For example, mean litter sizes of cubs and yearlings in Alaska were 1.63 and 1.49, respectively (Amstrup 1995). In Svalbard, these values were 1.81 and 1.32, respectively, whereas litter sizes of polar bears in Hudson Bay during the early 1980s were 1.9 and 1.7 for cubs and yearlings, respectively (Ramsay and Stirling 1988, Derocher and Stirling 1992). Annual litter production rates as high as 0.45 litters/female have been reported for polar bears in the Hudson Bay area (Derocher and Stirling 1992). Nearly half of the females in that population were annually producing a litter of cubs at that time. By comparison, only one fourth of the female polar bears in the Beaufort Sea produce a litter of cubs each year (a litter production rate of 0.25) (Amstrup 1995). That is, in Hudson Bay, each female had a litter nearly every other year, but in the Beaufort Sea, each female produced a litter only every fourth year. Because polar bears in Hudson Bay also produced larger litter sizes, these differences in litter production rates translated into a much higher overall reproductive rate there than in the Beaufort Sea. Female polar bears in the Beaufort Sea produced only ~ 0.40 cub/year, whereas in the Hudson Bay area they produced up to 0.90 cub/year at the time those studies were conducted (Derocher and Stirling 1992). Reproductive rates in most other areas appear to be more similar to those in the Beaufort Sea than in Hudson Bay.
In assessing reproductive intervals, it is critical to confirm weaning, as opposed to mortality of cubs. Many polar bear cubs die in their first year of life (Amstrup and Durner 1995). Those females can breed again in the year of the loss (if it occurs early enough in the spring) or the next year. The breeding frequency, by itself, might suggest a short reproductive interval when it is actually prolonged by poor cub survival. In addition to documenting that tagged females were no longer accompanied by yearling cubs in the spring, Ramsay and Stirling (1988) also captured many weaned yearlings in the autumn of their second year (approximately 1.8 years of age), confirming that many females in the Hudson Bay region actually did have a 2-year reproductive interval.

Lønø (1970), Stirling et al. (1977b), and Ramsay and Stirling (1988) reported on populations that may have been well below carrying capacity due to unregulated hunting (Stirling et al. 1977b; Larsen 1986; Derocher and Stirling 1992, 1995a). Likewise, breeding intervals in the Hudson Bay area have increased, possibly in response to increased relative density of bears in the area (Derocher and Stirling 1992, 1995b). Annual litter production rate in the Hudson Bay region declined from 0.45 litter/female in the period from 1965–1979 to 0.35 during 1985– 1990 (Derocher and Stirling 1992). A higher proportion produced cubs every 3 years in the latter period. The inverse of the litter production rate is the interbirth interval. That increased from 2.22 years in 1965– 1979 to 2.86 years in 1985–1990. Simultaneously, cub mortality from spring to autumn was significantly higher in the latter period (Derocher and Stirling 1992). The proximate factor associated with all of these trends was the declining weight of adult females during this 25-year period (Derocher and Stirling 1992). Age of first successful reproductive effort increased, although pregnancy rates did not change noticeably. An increasing age of maturation may indicate that a population is approaching carrying capacity. Age of maturation in mammals is often associated with attainment of a threshold body mass (Sadleir 1969) which could be more difficult to attain as competition for resources increases.
A delay in reaching that threshold mass may signal density dependent influences on the population. Such influences, however, also could result from environmental changes that reduce carrying capacity rather than from increases in polar bear numbers. The documented declines in body weights of females, declines in numbers of independent yearlings, and protracted reproductive intervals appear to be closely related to earlier deterioration of the sea-ice of Hudson Bay (Stirling etal.1999).Thesea-ice extent in the Arctic has been declining throughout the past two decades (Gloersen and Campbell 1991; Vinnikov et al. 1999). Declining Arctic sea-ice cover by itself is difficult to link with polar bear reproductive performance. The timing of melt of the sea-ice in Hudson Bay, however, is more easily connected. Polar bears there, especially pregnant females, depend heavily on the spring and early summer foraging for seals to carry them through the ice-free period (late summer to autumn). Pregnant females, unlike other polar bears in Hudson Bay, remain ashore in autumn when ice returns, and may be food deprived for up to 8 months. Those females must secure sufficient fat stores during the spring and summer to see them through that long period of food deprivation (Stirling 1977; Derocher and Stirling 1992). The mean date of sea-ice break-up in the late 1990s was >2 weeks earlier than it was in the 1970s and early 1980s (Stirling et al. 1999). Earlier break-up and the shortened foraging period accompanying it may mean a significant reduction in the fat stores female polar bears can accumulate before denning. This hypothesis is strengthened by the observation of a transient increase in condition of females coming ashore during the early 1990s when cooler than normal temperatures resulted in later break-ups (Stirling et al. 1999).
Evidence of the critical link between availability of seal prey and reproduction in polar bears is also available in more northerly parts of the range. Weights of females and their reproductive out put in the Beaufort Sea decreased markedly in the mid-1970s following a decline in ringed and bearded seal populations (Stirling et al. 1976, 1977b; Kingsley 1979; DeMaster et al. 1980; Stirling et al. 1982; Amstrup et al. 1986). The strength and longevity of declines in reproductive parameters varied both geographically and temporally with the severity of ice conditions that reduced numbers and productivity of seals (Amstrup et al. 1986).
Global warming. The Fourth Assessment Report of the Intergovernmental Panel on Climate Change of the United Nations, published in January 2007, came to the “unequivocal” conclusion that the world’s climate is warming rapidly and that the primary cause is human activity. What this means for polar bears, is that large-scale loss of ice will continue, with severe negative impacts on the bears’ survival, reproduction, and population size. The effects of climate warming will vary in timing and rate of change in different regions but, over the long term, the effects will be negative.

An adult female and her cub on shore in Svalbard. The ice is gone so now, like the bears in summer in Hudson Bay, they must live on her stored fat reserves for several months before they are able to return to the sea ice to hunt seals, a critical season witch duration may increase with global warming effects on arctic sea ice - © Ian Stirling.
In Hudson Bay-Foxe Basin and in the eastern Canadian Arctic (Baffin Bay and Davis Strait-Labrador Sea), the sea ice melts completely each summer. Since the most important feeding period for bears there is from mid-April until breakup, they are already among the first populations to be negatively affected by climate warming. Bears there survive the summer mainly on their stored fat, although they scavenge opportunistically, sometimes feed on vegetation, and may attempt to hunt other marine mammals. However, polar bears obtain almost all their annual energy requirements by hunting seals from the sea ice surface, not from other sources. Speculation that polar bears might sustain themselves from alternate food sources on land is fanciful.
The main area where multiyear ice presently remains in late summer and fall, and will in future, is along the coastline and between some of the most northerly islands of Canada and Greenland. If the relatively unproductive multiyear ice there is replaced by annual ice, over the continental shelf at least, it is possible that the biological productivity, and its suitability for seals and polar bears, may increase, at least in the short-term. However, if the climate continues to warm unchecked, as is currently predicted, this possible respite may be brief.

Despite the enormous changes in the Arctic that have already been documented, humans still can and must respond. It will likely be several decades before the most negative predictions are realized. Thus, time is critical for both polar bears and the sea ice that dominates the Arctic’s marine ecosystem. Polar bears evolved into existence because of a large productive habitat, unoccupied by a terrestrial predator. As that habitat disappears, so will the bears that live and depend on it. It is vital that all humans use whatever time remains to reduce greenhouse gas emissions sufficiently for sea ice and polar bears to persist for our children and grandchildren to marvel at. Nothing less is defensible.
Conservation History
In the 1960s and 1970s, hunting was the major threat to polar bears. Pressure from hunters was so great, and their survival in such jeopardy, that the five polar bear nations reached a landmark accord.
The International Agreement on the Conservation of Polar Bears was signed in Oslo, November 15, 1973 by: Canada, Denmark (Greenland), Norway, the U.S., and the former U.S.S.R. It is one of the first and most successful international conservation measures enacted in the 20th century.
The Agreement
* Prohibited random, unregulated sport hunting of polar bears
* Outlawed hunting from aircraft and icebreakers, a common practice
* Required each nation to protect polar bear denning areas and migration patterns
* Obliged the nations to conduct research on the conservation and management of polar bears to share research findings with each other
Today, member scientists from each nation continue to work together to address new threats including climate change, pollution, industrial activities, and poaching. They meet every three to four years under the auspices of the IUCN World Conservation Union to coordinate research.

Figure 5. George Durner, USGS research zoologist, examines a 1240 pound adult male captured 65 miles off the Chukchi sea coast in April, 2008.
Current Status
As of May 2008 the U.S, lists the polar bear as a threatened species under the Endangered Species Act. Russia considers the polar bear a species of concern.
What’s happening? Today, scientists have concluded that the threat to polar bears is ecological change in the Arctic from global warming. Polar bears depend on sea ice for hunting, breeding, and in some cases, denning. Summer ice loss in the Arctic now equals an area the size of Alaska, Texas, and the state of Washington combined.
Polar bears range from Russia to Alaska, from Canada to Greenland, and onto Norway's Svalbard archipelago—the five polar bear nations. Biologists estimate there are 20,000 to 25,000 bears. About 60% of those live in Canada.
At the 2009 meeting of the IUCN Polar Bear Specialist Group, scientists reported that of the 19 subpopulations* of polar bears:
* 8 are declining
* 3 are stable
* 1 is increasing
By comparison, in 2005:
* 5 were declining
* 5 were stable
* 2 were increasing
*Insufficient data to determine the fate of the other 7 populations

References
Amstrup, C. Steven. 1988. Polar Bears (Chapter 27) in Wild Mammals of North America.
PDF: www.polarbearsinternational.org/sites/default/files/pdf/PolarBearsComprehensive.pdf
Bechshøft, Thea Ø, Sonne, Christian, Rigét, Frank F, Wiig, Øystein and Dietz, Rune. 2008. Differences in growth, size and sexual dimorphism in skulls of East Greenland and Svalbard polar bears (Ursus maritimus).
Derocher, A. E. and Ø. Wiig. 2002. Postnatal growth in body length and mass of polar bears (Ursus maritimus) at Svalbard.
Kolensky, B. George. 1987. POLAR BEAR (Chapter 37) in Wild Furbearer Management and Conservation in North America.
Manning, T. 1971. Geographical variation in the polar bear Ursus maritimus Phipps.
Polar Bears International: www.polarbearsinternational.org/polar-bears/bear-essentials-polar-style/conservation
Stirling, Ian. Polar Bears. 1988. University of Michigan Press Ann Arbor.
- Ours blanc: chronique d'une extinction annoncée: www.lecerclepolaire.com/articles_archives/Stirling-Polar_Bear.html
WWF: www.worldwildlife.org/species/finder/polarbear/polarbear.html
PDF LINKS ANEX:
www.usgs.gov/newsroom/special/pol....dyCondition.pdf
www.cnr.uidaho.edu/wlf314/lecture_notes/Rode_etal_2010.pdf
General Polar Bear discussion thread:
shaggygod.proboards.com/index.cgi?action=display&board=northamericanpolarbear&thread=644&page=1






